Moving your chemotherapy regimen forward is critical
Severe and febrile neutropenia can cause chemotherapy dose delays, reductions, and interruptions1-4
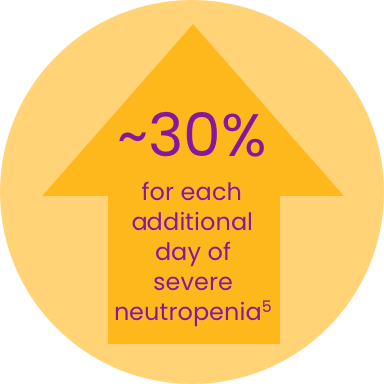
- Longer duration of severe neutropenia is associated with increased risk of progression to febrile neutropenia5
- Risk of infection-related hospitalization increases by 28-30% for each additional day a patient has grade 3 or grade 4 neutropenia in the first cycle5
- 1 in 5 chemotherapy patients are at risk for severe neutropenia in cycle 16
Incremental risk of hospitalization
Severe neutropenia: absolute neutrophil count (ANC) <500/µL7
Increased risk of serious infection7
Potential chemotherapy dose delays, reductions, and interruptions1,8
ROLVEDON® was studied in 2 Phase III clinical trials.
National Comprehensive Cancer Network® (NCCN®) Guidlines9*:
Basis for NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Regarding MGF Usage
- Current NCCN guidelines recommend assessing risk of FN before every cycle.
- Evaluate the risk of FN following chemotherapy in adult patients with solid tumors and non-myeloid malignancies.
- Administer primary G-CSF prophylaxis in first and subsequent cycles for patients at high or intermediate FN risk.
- For reference, see MGF-1 in the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Hematopoietic Growth Factors.
*NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way.
References
- Lyman GH. Impact of chemotherapy dose intensity on cancer patient outcomes. Natl Compr Canc Netw. 2009;7(1):99-108.
- Lyman GH, Dale DC, Friedberg J, Crawford J, Fisher RI. Incidence and predictors of low chemotherapy dose-intensity in aggressive non-Hodgkin’s lymphoma: a nationwide study. J Clin Oncol. 2004;22(21):4302-4311.
- Denduluri N, Patt DA, Wang Y, et al. Dose delays, dose reductions, and relative dose intensity in patients with cancer who received adjuvant or neoadjuvant chemotherapy in community oncology practices. J Natl Compr Canc Netw. 2015;13(11):1383-1393.
- Schwartzberg LS, Bhat G, Peguero J, et al. Eflapegrastim, a long-acting granulocyte-colony stimulating factor for the management of chemotherapy-induced neutropenia: results of a phase III trial. Oncologist. 2020;25(8):e1233-e1241.
- Li Y, Klippel Z, Shih X, Reiner M, Wang H, Page JH. Relationship between severity and duration of chemotherapy-induced neutropenia and risk of infection among patients with nonmyeloid malignancies. Support Care Cancer. 2016;24(10):4377-4383.
- Lyman GH, Kuderer NM, Crawford J, et al. Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy.Cancer. 2011;117(9):1917-1927.
- Braden CD. Neutropenia. Medscape website. https://emedicine.medscape.com/article/204821-overview#showall. Accessed June 17, 2020.
- Crawford J, Dale DC, Kuderer NM, et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Canc Netw. 2008;6(2):109-118.
- Referenced with permission from the National Comprehensive Cancer Network, Inc. © National Comprehensive Cancer Network, Inc. 2020. All rights reserved. Accessed May 5, 2022. To view the most recent and complete version of the recommendations, go online to NCCN.org.
ROLVEDON® was studied in 2 Phase III clinical trials.