The first long-acting myeloid growth factor in over 20 years with a unique molecular structure1
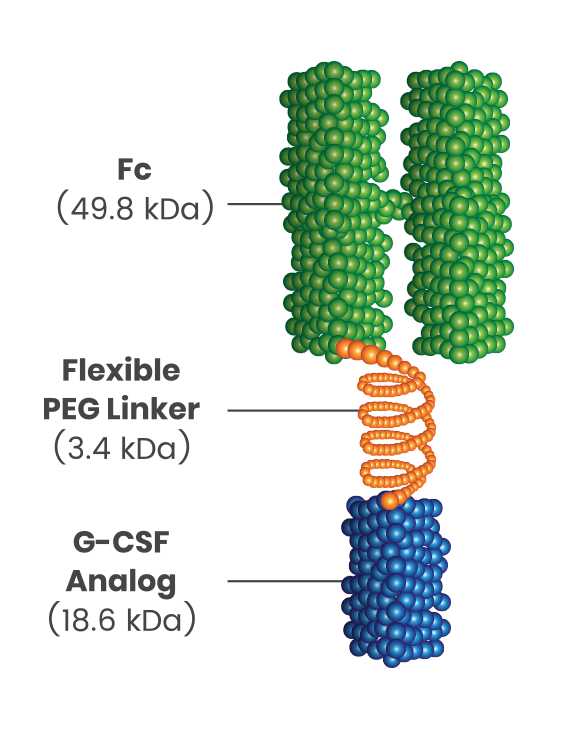
Introducing ROLVEDON®:
- Novel formulation combines a granulocyte colony-stimulating factor (G-CSF) analog with an Fc fragment of human immunoglobulin G4 (IgG4)2
- Supported by a complete innovator biopharmaceutical development program
Selected Safety Information
Serious Allergic Reactions
- Serious allergic reactions, including anaphylaxis, can occur in patients receiving rhG-CSF products. Permanently discontinue ROLVEDON in patients who experience serious allergic reactions.
FcRn facilitates ROLVEDON transcytosis and recycling3
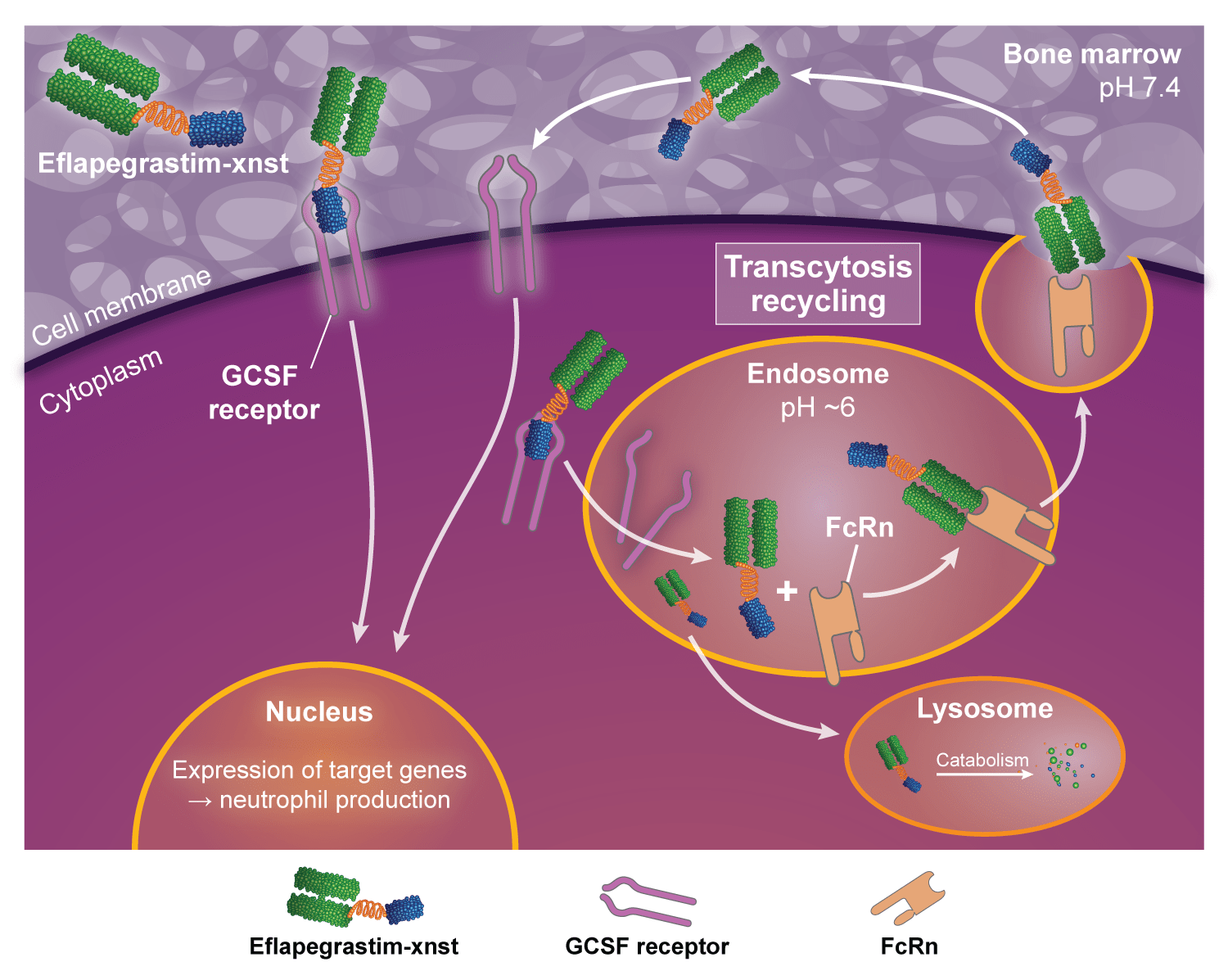
- It has been shown in:
- in vitro studies2:
- To bind the neonatal Fc receptor (FcRn), which facilitates transcytosis
- in vivo animal studies3:
- To facilitate uptake and retention of G‑CSF in the bone marrow
- in vitro studies2:
- The clinical significance of these findings is unknown
Watch MOA video
Eflapegrastim‑xnst is a recombinant human granulocyte growth factor that binds to G-CSF receptors on myeloid progenitor cells and neutrophils, triggering signaling pathways that control cell differentiation, proliferation, migration, and survival.2
MOA=mechanism of action.
References:
- Schwartzberg LS, Bhat G, Peguero J, et al. Eflapegrastim, a long-acting granulocyte-colony stimulating factor for the management of chemotherapy-induced neutropenia: results of a phase III trial. Oncologist. 2020;25(8):e1233-e1241.
- ROLVEDON [package insert]. Lake Forest, IL: Spectrum Pharmaceuticals, Inc.
- Barrett J, et al. Eflapegrastim's enhancement of efficacy compared with pegfilgrastim in neutropenic rats supports potential for same-day dosing. Experimental Hematology 2020;92:51-61.
ROLVEDON is administered via a single subcutaneous dose